how to draw molecular orbital diagrams for polyatomic molecules
Atomic orbitals are representations for where electrons are likely to be found. A fundamental principle of these theories is that as atoms bond to form molecules a certain number of atomic orbitals combine to form the same.
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook
An analysis of the molecular orbitals for the water molecule provides a.

. Molecular orbital mo diagram of polyatomic molecules beryllium dihydride beh2 and water h2o youtube a σ1s mo lower in energy than either of the 1s aos. Bonding in polyatomic molecules. In polyatomic molecules we can have more than two atoms combining eg.
F has two electrons in the 2s energy level and five electrons in the 2p orbitals. Roussel MOs for polyatomic molecules January 8 20201023. For example B has two electrons in the 2s orbital and one in the 2p orbital.
Draw out the MO diagram and label in the valence electrons. HF nb σ σ Energy H 136 eV 1s F 186 eV 402 eV 2s 2p So HF has one σ bond and three lone electron pairs on fluorine. Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube.
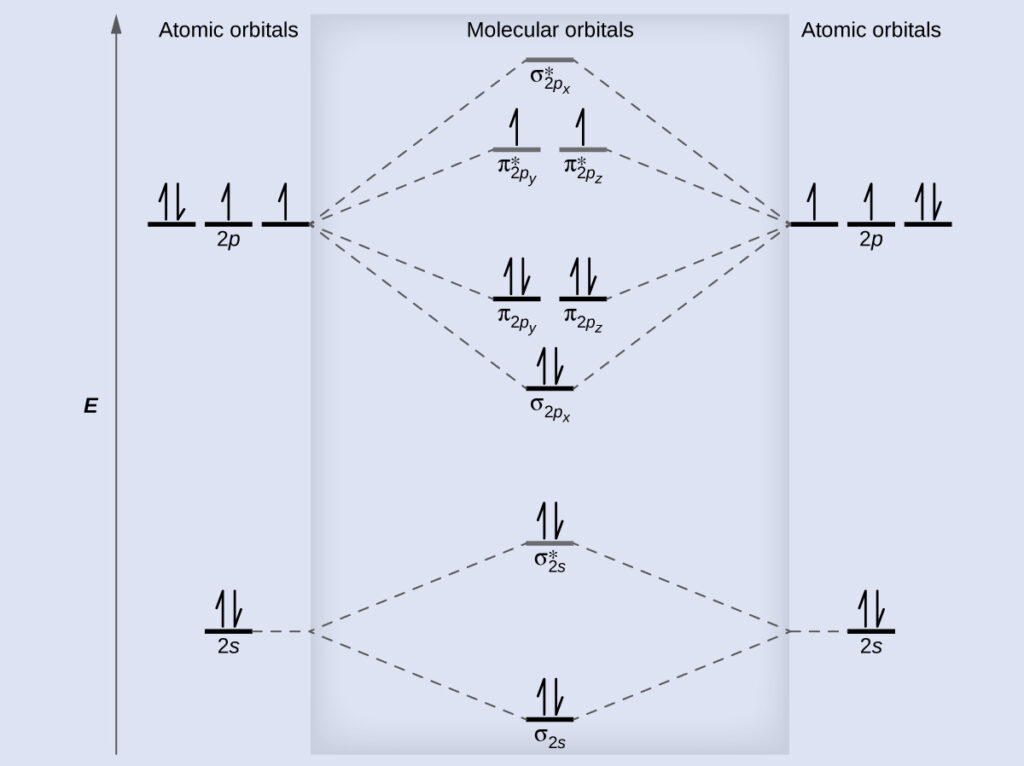
Molecular Orbital Theory Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals. All of the orbital filling principles Hunds Rule Pauli Exclusion Principle Aufbau Principle.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The core premises of this method are. In most cases this means that molecules tend to adopt the geometry that maximises the.
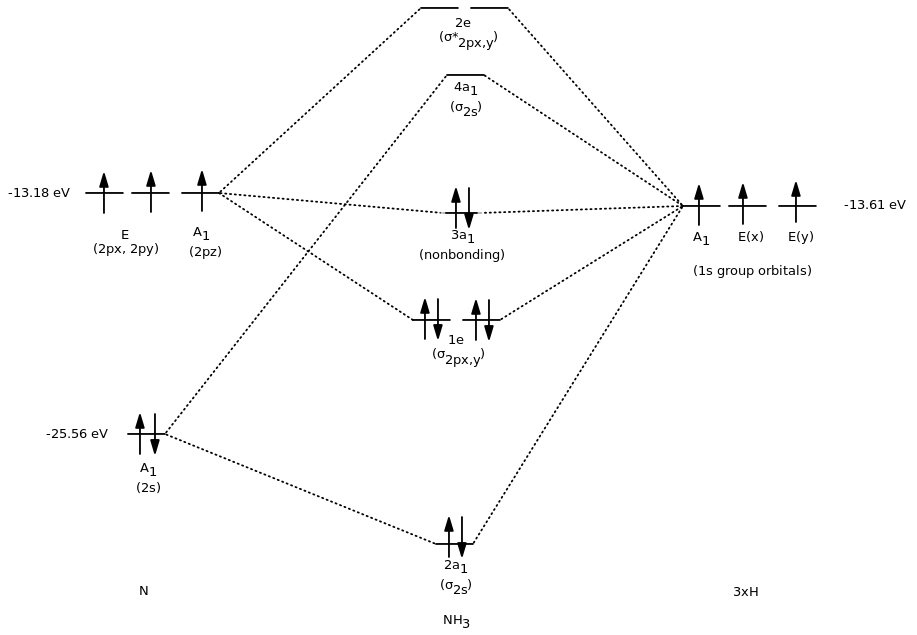
Step 3 Fill in the electrons in the correct MO diagrams molecular orbitals. From the geometry of the molecules as predicted by VSEPR we can deduce the hybridization of the central atom. Each hybrid orbital is composed of a combination of an s and a p orbital on the central atom.
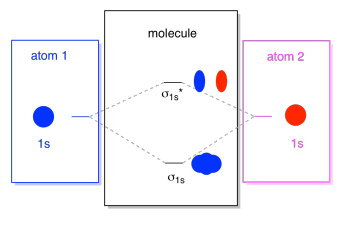
A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO method in particular. In case of beryllium hydride there are 3 atoms overlapping simultaneously. 8 4 molecular orbital theory chemistry.
In general a molecular orbital in a polyatomic system extends over all the nuclei in a molecule and it is essential if we are to understand and predict the spatial properties of the orbitals that we make use of the symmetry properties possessed by the nuclear framework. Molecular Orbital Theory for Larger Polyatomic Molecules. The numbers and shapes of the orbitals depend on.
The way these bonds are placed on any molecular orbital diagram is according to how the atomic orbitals that make the mos mix. Co in molecules with more than one type of atom mos are formed from aos that have different energies. Molecular Orbital Diagrams for Linear Polyatomics Answers 193 KB.
In this video qualitative MO diagrams will be generated for simple polyatomic molecules water ammonia and difluorocarbene using so-called ligand group o. 2 Orbital hybridization - sp Hybrid orbitals generated by mixing the characters of atomic orbitals 2 1 ψsp_hybrid ψ2s. Sp3_hybrid2 s xpz 1.
So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each. Because the and ˇ orbitals arise from independent LCAOs we can build the ˇ orbital energy diagram independently of the orbitals. MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works.
Ψmo c1 ψ1 ao c2 ψ2 ao. Each atom surrounding a central atom is considered to have its own set of axes. Step 3 Fill in the electrons in the correct MO diagrams molecular orbitals.
Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube A σ1s MO lower in energy than either of the 1s AOs. An analysis of the molecular orbitals for the water molecule provides a. Draw out the mo diagram and label in the valence electrons.
In this video we create a molecular orbital diagram for peroxide and enter it into the ALEKS homework system. Based on the amount of orbital overlap the relative changes in energy differ going from the atomic orbital to the molecular orbital. Draw the orbital diagram for ion Co 2.
First step is to determine which MO diagram were using. Use the diagram to predict properties of the molecule. If the HOMO is unperturbed by the structural change under consideration then the occupied MO lying closest to it governs the geometric preference.
In case of beryllium hydride there are 3 atoms overlapping simultaneously. Molecular Orbitals for Polyatomic Molecules. Hybrid orbitals describe the bonding in polyatomic molecules one bond at a time.
Not spheres pointing towards the central atom are considered to be along the y axis rather than the z axis. ψ ψ ψ ψ ψ. So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0.
In a molecule with more than two atoms any orbitals with directional aspects ie. Linear molecules are sp hybridized. For molecules with nice Lewis diagrams octet rule satis ed that have multiple bonds the frontier orbitals are very frequently ˇ orbitals.
Individual atomic orbitals AO are arranged on the far left and far right of the diagram. This is the general mo diagram you need to fill with the valence electrons of bn boron has 3 valence electrons and nitrogen has 5 valence electrons this makes 8 electrons. Carbon dioxide CO2 molecule is triatomic and linear like Beryllium di hydride BeH2 However unlike hydrogen as peripheral atoms in BeH2 there are oxyge.
Walsh diagrams Walshs rule A molecule adopts the structure that best stabilises the HOMO. Draw the orbital diagram for ion Co 2. Greater overlap greater change in.
In this case the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory Walsh diagram Water 1045 X H H H O H. The F 2s is nonbonding.

Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

Cbr4 Lewis Structure Molecular Geometry Polarity Hybridization Bond Angle Molecular Geometry Molecular Shapes Molecular
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts
3 3 4 Assembling A Complete Mo Diagram Chemistry Libretexts

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube
Molecular Orbital Theory For Polyatomic Molecules Socratic

Name Of Elements With Atomic Number Atomic Mass Valency Science Notes Chemistry Notes Biology Notes

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

Molecular Orbitals Molecular Orbitals For Polyatomic Molecules
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

Molecular Orbital Mo Diagram Of Polyatomic Molecules Beryllium Dihydride Beh2 And Water H2o Youtube

Mo Diagram Overview How To Draw Mo Diagram And Solved Example Along With Faqs

Frontier Molecular Orbitals For Reaction Of H 2 O With Co 2 The Shapes Download Scientific Diagram

5 4 Larger Polyatomic Molecules Chemistry Libretexts
8 7 Molecular Orbitals For Polyatomic Molecules Chemistry Libretexts

Lewis Dot Structures Worksheet Cheat Sheet Math Addition Worksheets Molecular Geometry Worksheet Template

8 4 Molecular Orbital Theory Chemistry

Lewis Dot Structures Worksheet Cheat Sheet Math Addition Worksheets Molecular Geometry Worksheet Template